SOURCE :
A study to evaluate the efficacy and safety of SanAshwa in the management of stress and anxiety in healthy adult volunteers
Prospective, Randomized, Double-Blind, Placebo controlled Human Clinical Study using SanAshwa
Table: Mean Demographic data of the study subjects
| Supplement Groups | Female | Male | Total subject in each Supplement group |
|---|---|---|---|
| SanAshwa | 15 | 20 | 35 |
| Placebo | 13 | 22 | 35 |
| Total | 28 | 42 | 70 |
INCLUSION CRITERIA: The subjects were included based on following criteria
- Male or female, age 18 -50 years
- Healthy volunteers who are willing to give informed consent
- Healthy volunteers who are able to comply with the study requirements
- anxiety and depression score equal or below 12 and above 4
- Subjects with WHO-5 wellbeing index < 15 or PSS scores > 14
- Standard safety biology.
EXCLUSION CRITERIA: The subjects were excluded based on following criteria
- Pregnancy
- Any current ongoing medical illness
- Neurologic or psychiatric pathology,
- Consumption of psychotropic
- High level of caffeine consumption
- Any important chronic pathology
- Drugs which impairs concentration, anxiety and stress
- HAD-A results above 12 and below 4
TOTAL TREATMENT DAYS: 28 Days
SERVINGS PER DAY: 2 Capsules – One after breakfast and one after dinner
Primary Endpoints
- Evaluation of changes in the Serum Cortisol levels from Screening to EOT
- Evaluation of the changes in Stress sub-scale of Depression Anxiety and Stress Scale-21 (DASS-21) from Screening to EOT
- Evaluation of the changes in the7-item sub-scale measuring symptoms of stress.
- Evaluation of the changes in the Patient Health Questionnaire-9 (PHQ-9) from Screening and EOT
- Evaluation of the changes in the Generalized Anxiety Disorder-7 (GAD-7)
Secondary Endpoints
- The safety and tolerability were assessed by observation and by volunteered reports from the patients. Adverse events were documented with the concomitant medications.
Statistical Methods
The data was analyzed with 5% significance level and 80 % power for study using SAS. The difference between the groups was assessed using paired t-test for Serum Cortisol levels and Wilcoxon Rank Sum Test was used to assess difference between the groups for rest of the parameters.
Ethical Conduct of the study
The study was initiated after written approval from Institutional Ethics Committee and also after registration of this trial in CTRI. The trial was conducted as per the ICMR Guidelines for Biomedical Research on Human subjects, ICH GCP Guidelines, Schedule Y, Declaration of Helsinki (Brazil, 2013) and in accordance with other applicable guidelines.
EVALUATION OF CHANGES IN THE SERUM CORTISOL LEVELS
Table: Mean Serum Cortisol level µg/dLat screening and EOT
| Groups | Baseline (N=35) | EOT- Day 28 (N=35) | P-value* |
|---|---|---|---|
| SanAshwa (Supplement A) | 12.34371429 ± 4.183 | 7.943714286 ± 5.710 | 0.0003 |
| Placebo (Supplement B) | 11.02629 ± 4.273068 | 11.65857 ± 4.972356 | 0.5764 |
| P-value# (Supplement A vs Supplement B ) |
0.1968 | 0.0050 |
Figure: Mean Serum Cortisol level at screening and EOT:
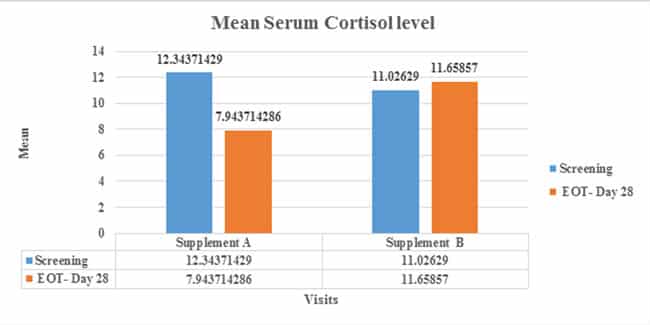
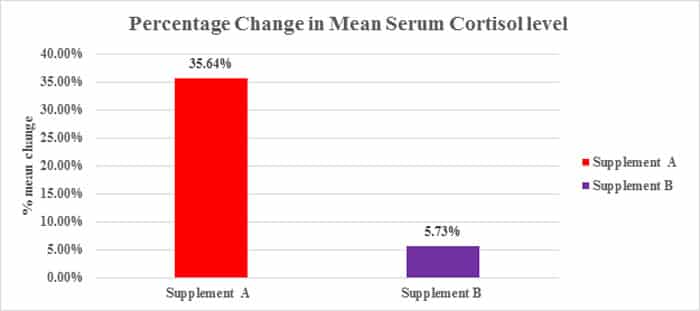
Table: Percentage change in Serum Cortisol level from screening to EOT
| Group | Screening to EOT( Day 28) |
|---|---|
| SanAshwa (Supplement A) | 35.64 % |
| Placebo (Supplement B) | 5.734 % |
Figure: Percentage change in mean change of Serum Cortisol level
EVALUATION OF THE CHANGES IN STRESS SUB-SCALE OF DEPRESSION ANXIETY AND STRESS SCALE-21 (DASS-21)
Table: Percentage Change in Mean of Stress subscale of depression anxiety and stress from the screening to EOT
| Change mean | SanAshwa (Supplement A) |
Placebo (Supplement B) |
||||
|---|---|---|---|---|---|---|
| Stress | Anxiety | Depression | Stress | Anxiety | Depression | |
| Screening to EOT | 18.78 % | 21.84 % | 18.69 % | 11.10 % | 9.17 % | 10.75 % |
Figure: Percentage Change in Mean of Stress subscale of depression anxiety and stress from the screening to EOT
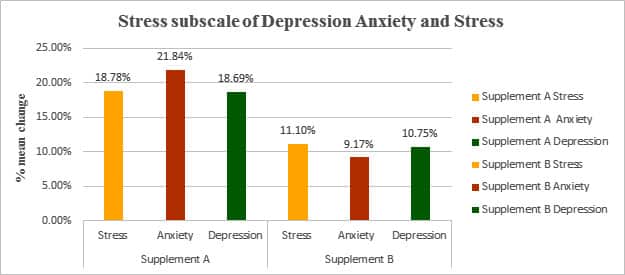
*The DASS-21 questionnaire is a quantitative measure of distress on the basis of three subscales of depression, anxiety (e.g., symptoms of psychological arousal) and stress (e.g., cognitive, subjective symptoms of anxiety).
EVALUATION OF THE CHANGES IN THE 7-ITEM SUB-SCALE MEASURING SYMPTOMS OF STRESS
Table: Percentage Change in Mean of symptoms of stress from the screening to EOT
| % Change mean | SanAshwa (Supplement A) |
Placebo (Supplement B) |
||
|---|---|---|---|---|
| Anxiety | Depression | Anxiety | Depression | |
| Screening to EOT | 28.46 % | 31.26 % | 15.39 % | 13.69 % |
Figure: Percentage Change in Mean of symptoms of stress from the screening to EOT
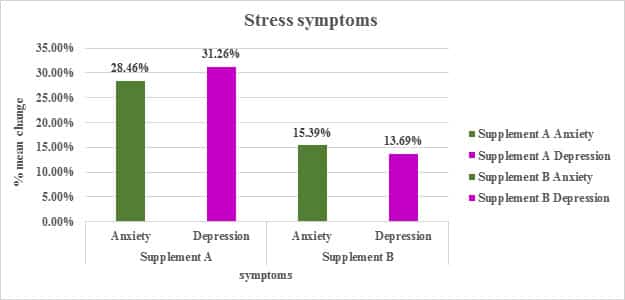
*The Perceived Stress Scale (PSS) is the most widely used psychological instrument for measuring the perception of stress.
EVALUATION OF THE CHANGES IN THE PATIENT HEALTH QUESTIONNAIRE-9 (PHQ-9)
Table: Percentage Change in Mean of Depression from the screening to EOT
| % Change mean | SanAshwa (Supplement A) |
Placebo (Supplement B) |
|---|---|---|
| Depression | Depression | |
| Screening to EOT | 26.18 % | 10.35 % |
Figure: Percentage Change in Mean of Depression from the screening to EOT
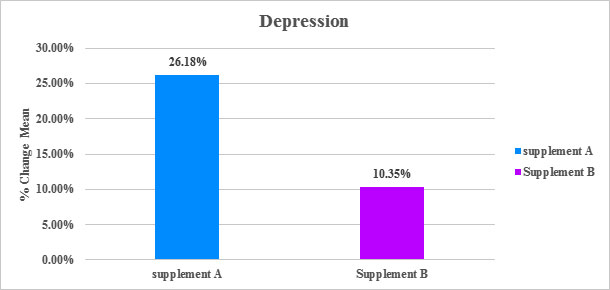
*The PHQ-9 (Patient Health Questionnaire-9) objectifies and assesses degree of depression severity via questionnaire.
EVALUATION OF THE CHANGES IN THE GENERALIZED ANXIETY DISORDER-7 (GAD-7)
Table: Percentage Change in Mean of Anxiety from the screening to EOT
| % Change mean | SanAshwa (Supplement A) |
Placebo (Supplement B) |
|---|---|---|
| Anxiety | Anxiety | |
| Screening to EOT | 31.22 % | 12.32 % |
Figure: Percentage Change in Mean of Anxiety from the screening to EOT
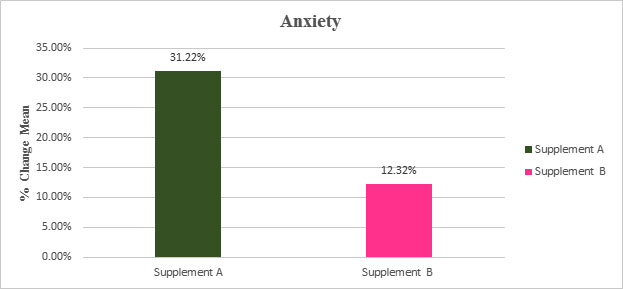
*The GAD-7 (General Anxiety Disorder-7) measures severity of anxiety
SanAshwa is SAFE & EFFECTIVE
Results obtained from Statistical and Efficacy analyses of the clinical study in healthy subjects, compared to the placebo, indicated SanAshwa is effective in lowering the
- Serum Cortisol Levels,
- Stress scores,
- Anxiety scores and
- Depression scores


